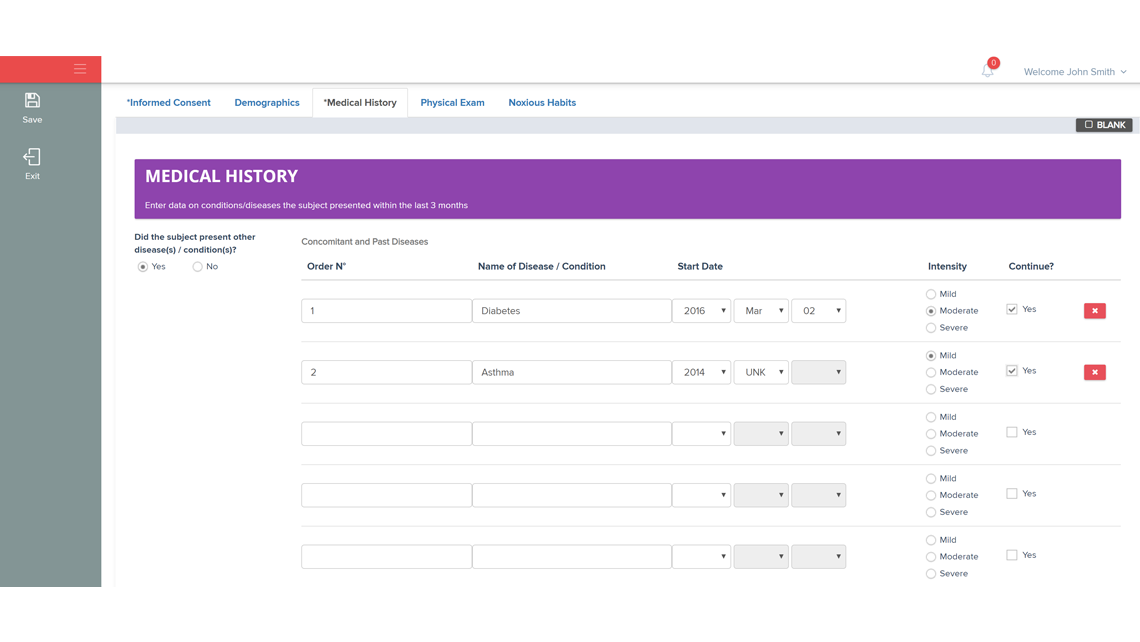
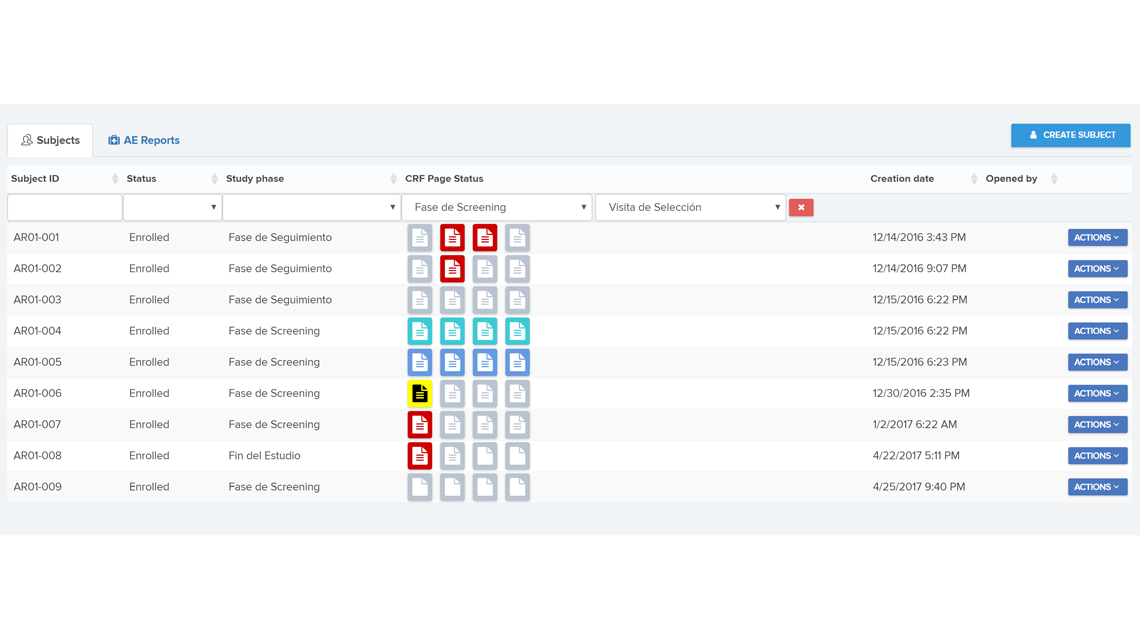
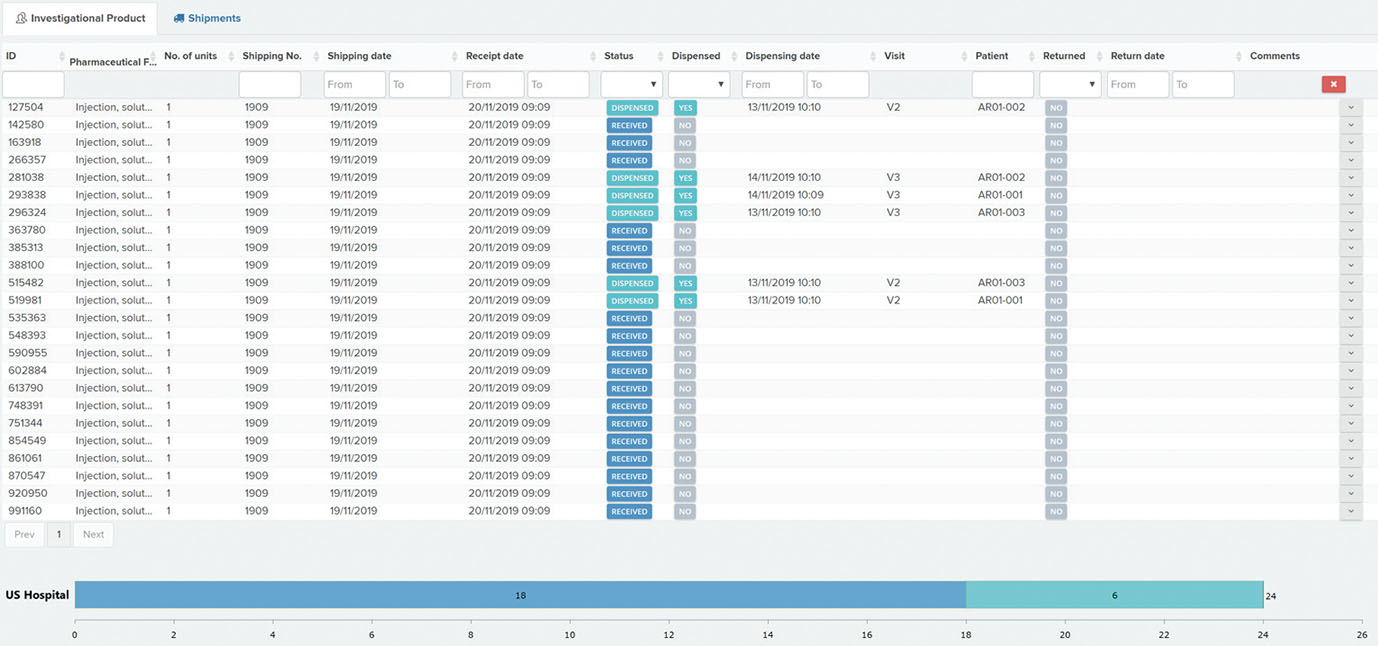
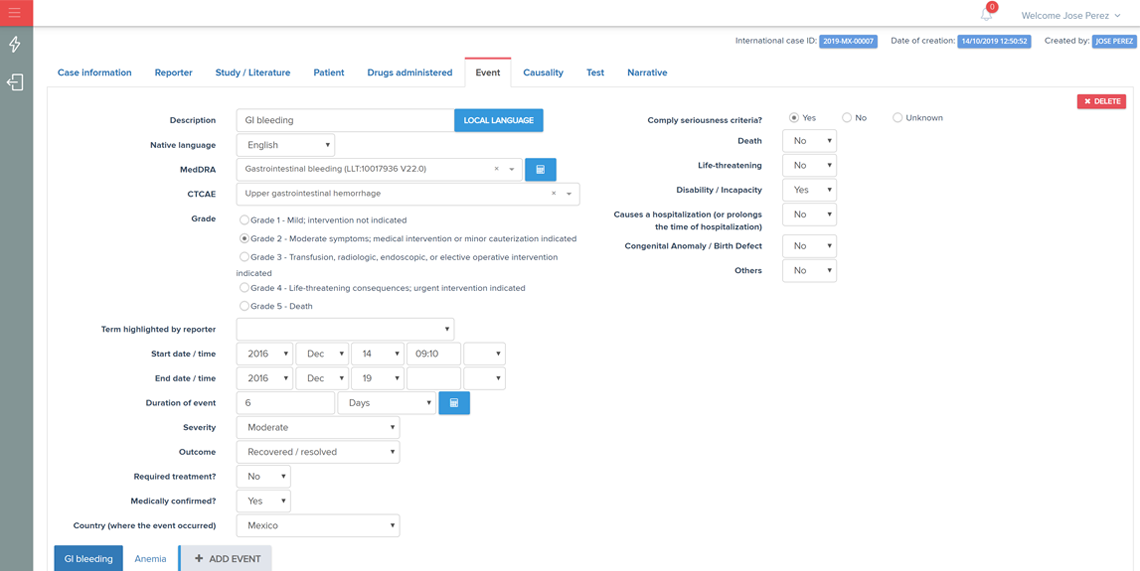
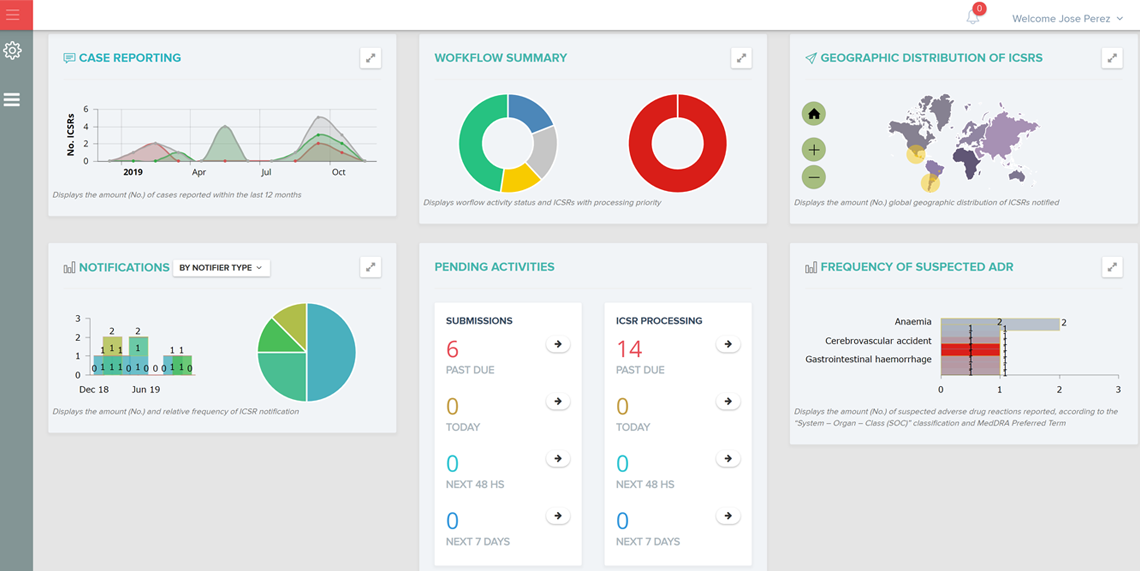
A user-friendly electronic case report form (eCRF) / study database application and infrastructure aimed for an “end-to-end” management of the clinical data from phase I to IV clinical studies (collection, review, coding and reporting).
The application was designed according to investigational team needs and based on years of experience in reporting/monitoring clinical data.